In this post, we will delve into the genetic underpinnings of PDE, shedding light on the diverse types of mutations associated with PDE.
Genetic Basis of PDE:
Pyridoxine-Dependent Epilepsy is primarily a genetic disorder, which means it arises from alterations in a person’s genes. Specifically, it is an autosomal recessive genetic condition, meaning that an affected individual needs to inherit two copies of the mutated gene, one from each parent in order to show symptoms of the conditions. The mutations that lead to PDE are within the Antiquitin (ATQ) gene (also known as ALDH7A1) which encodes …protein.
Primer on Proteins:
Biology is a complex topic, and perhaps you have never had to think of protein before outside of nutrition labels and cookouts. In fact, proteins are a LARGE group of molecules in our body that can combine together to make muscle (the protein we eat as steak), send signals, and contribute to a host of other very important functions. It is estimated that there are more than 100,000 different proteins with multiple variants of each in the human body! They are not the only molecules in our body, there are also hormones, fats, and carbohydrates which all come together in a network to make us who we are and allow us to function.
Antiquitin, or the protein implicated in many genetic cases of PDE, is a type of protein known as an enzyme. As a general category, enzymes help facilitate reactions in the human body. Sometimes it is hard to make sense of how one protein can have such a big effect on our children, but in fact this is often the case. The reason for this is that one protein can participate in a pathway that has many offshoots and consequences.
The ALDH7A1 Gene:
The gene responsible for PDE is known as Antiquitin, or ALDH7A1, located on chromosome 5. This gene encodes an enzyme called antiquitin (also known as alpha-aminoadipic semialdehyde dehydrogenase). Antiquitin is crucial for the breakdown of certain amino acids, including lysine and tryptophan, and the synthesis of vitamin B6 (pyridoxine) in the body.
Primer on Genetics:
The genetic code is the instruction manual to making us who we are. Just like there are only a certain amount of letters in each language which allow us to write a book, there are only 4 letters which comprise the code of genetics which are called bases. The bases are A, T, C, and G. As well, although the words in a language can be of variable length composed of known letters, all words in your DNA are composed of 3 bases and these three words are called codons. Examples of codons are ATT, CAG, GTC, etc. As all words are put on a page which fits into a book, the genetic code of codons is put into individual chromosomes which can be called chapters. There are 23 sets of chromosomes in each person. One set from each parent: 23 from mom and 23 from dad make for 46 chromosomes. You can actually see chromosomes under a microscope and a whole discipline dedicated to studying chromosomes called cytogenetics.
To us, each word in a book represents something we can understand. To the human body, each codon also translates to a signal in the form of an amino acid. Amino acids are actual ‘products’ of the genetic code and are used for manufacturing of all necessary proteins of the body and many other functions.
These codons put together create `sentences` or rather complete thoughts that the body can understand as messages. For example, many codons together can signal to the body that is should produce a hormone necessary, or another protein such as Antiquitin.
When random genetic changes happen, they can disrupt this code and although some words (codons) are left untouched, the change of one letter in one word can change the meaning of the whole sentence. These genetic changes are called genetic mutations.
Examples of genetic mutations are below:
Since we have previously discussed how one protein can have a large effect on the dynamics of a whole system, defects in the message of a protein due to these changes can cause interference in the growth and health of an individual. Imagine a concluding sentence in a book that no longer makes sense, and then the paragraph doesn’t seem to make sense, and although all the other words in the book are intact, the ending is not clear, and the book is now different.
In fact, in nature, mutations are not always harmful. Although the disruption of a gene seems like it would never be beneficial, sometimes it is. If we are speaking of human genetic conditions, mutations cause a change in the book of our DNA which underlies the medical issues that have been diagnosed. So, there is a change in the genetic DNA, which causes a change in codons, then amino acids, then in the final message, and the disruption in the message can cause the observed symptoms.
The genetic code is called the genotype of a person. The observed consequences are called the phenotype.
Types of Mutations in ALDH7A1:
There are more than 60 different known alterations in the ALDH7A1 gene which have been linked to PDE. Some mutations are more common than others. For example, 9 out of 60 are responsible for 61% of all known cases. The most common mutation is pGlu399Gln which is responsible for approximately 30% of all genetic causes of PDE.
In general, when a protein has been affected, two changes can occur. One is a gain of function when the new altered protein gains a new and usually harmful property. The other situation is a loss of function, when the altered protein is no longer able to do its previous job and it is this loss of ability which is harmful to the body. In PDE, the loss of function in the antiquitin protein (coded by the ALDH7A1 gene) has been linked to symptoms.
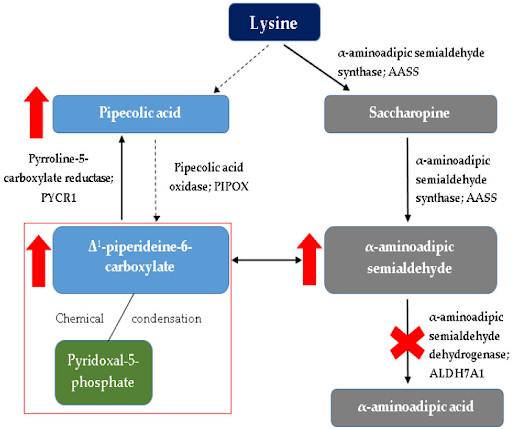
Explanation of the cascade that follows after loss of function of antiquitin.
As part of its many roles, Atiquitin acts in the breakdown of lysine. Lysine degradation has two pathways: one through the intermediate saccharopine leading to AASA and one through pipecolic acid leading to formation of P6P.
In a normal individual, then, AASA and P6P are in balance with each other and one form can convert to the other form. Here is where Antiquitin comes in. Antiquitin is supposed to take AASA and break it down to alpha aminoadipic acid (AAA), but when Antiquitin is unable to function due to a loss of function, this conversion does not take place.
Since AASA is no longer being converted to AAA, it builds up, and since it is able to convert to P6P, P6P also builds up and pipecolic acid is also increased to the back up of P6P. It is the same effect as with a clogged kitchen sink. When the sink stops draining (converting AASA to AAA), the water that is still running (since the body has a continual need to break down lysine) builds up.
From this build up, there are multiple reactions.
One is that the increase of P6P binds to PLP (which is the active form of Vitamin B6) and this binding leads to a chemical condensation or elimination of PLP (Vitamin B6) from the body. This is the actual mechanism behind the lack of vitamin B6 in PDE patients. By supplementing with oral vitamin B6, patients are able to compensate for this condensation of natural B6 and restore B6 dependent functions.
But as you can see from the diagram, restoring vitamin B6 levels does not take care of the increase in AASA and pipecolic acid. These compounds are now considered potentially neurotoxic and an explanation for why just vitamin B6 supplementation is not enough to prevent developmental delay.
Mutations in the ALDH7A1 gene can vary in type and severity, contributing to the clinical diversity observed in PDE patients. The specific mutation an individual carries can influence the severity and onset of PDE symptoms. Some mutations may lead to a complete loss of antiquitin function, resulting in severe seizures and developmental delays, while others may cause milder forms of the condition.
We will summarize publications related to specific mutations (ones you may have seen on a report from a doctor or geneticist in another blog).
Diagnosis and Genetic Testing
Diagnosing PDE often involves genetic testing to identify mutations in the ALDH7A1 gene. This genetic information is essential for tailoring treatment plans and understanding the potential impact on a patient’s health.
If you have any questions or would like to learn more about PDE genetics, please don’t hesitate to reach out. Let us know if you want a webinar on this topic or a group discussion. Together, we can make a difference in the lives of those affected by Pyridoxine-Dependent Epilepsy.
 Donate
Donate